The Element in Period 4 and Group 8
Period 5 Group 4. Carbon mentioned above is now considered Group 14 instead of Group 4A.
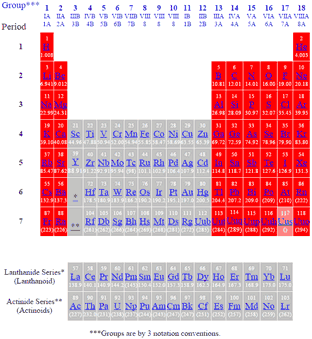
Periodic Table Of The Elements Main Groups
Helium is also found in.
. Period 4 elements start from potassium and end at krypton. For example the outer shell configuration of Li would be entered as 2 Submit Answer Try Another Version 1 item attempt remaining O segW. A new row is begun when chemical behaviour begins to repeat meaning that elements with similar behaviour fall into the same.
Iron is a Group 8B element in Period 4. Carbon mentioned above is now considered Group 14 instead of Group 4A. It contains the four elements titanium Ti zirconium Zr hafnium Hf and rutherfordium Rf.
Ase your answer to this question on the information below. Papers and texts published today have gone over to the newer nomenclature but sometimes we are using older literature. Hence we can find out the period 4 elements too.
So technically no element is in Group 4 period 2. Group 8A The Noble or Inert Gases. It consists of iron Fe ruthenium Ru osmium Os and hassium Hs.
Group 8A or VIIIA of the periodic table are the noble gases or inert gases. Zirconium the second element in Group 4 is in period 5 not period 2. Period 5 group 9.
Which element is in group 4 period 4. The element in period 4 and group 8 Other questions on the subject. A Group 8 element is one in the series of elements in group 8 IUPAC style in the periodic table which consists of the transition metals iron Fe ruthenium Ru osmium Os and hassium Hs.
Helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn. Period 5 group 1. Period 5 group 8.
A period 4 element is one of the chemical elements in the fourth row of the periodic table of the elements. Period 5 group 3. Chemistry 22062019 0020 paulawells11.
Group 4 is a group of elements in the periodic table. Chemistry 22062019 0150 lildestinyquintana. Express your answer as a series of outer shell orbitals.
This group lies in the d-block of the periodic table. 10 rows There are a total of 8 main group elements in period 4 of the periodic table. Group 8A or VIIIA of the periodic table are the noble gases or inert gases.
What are the spectator ions in 2h so42- ca2 2r caso4 2h 21. The period 4 transition metals are scandium Sc titanium Ti vanadium V chromium Cr manganese Mn iron Fe cobalt Co nickel Ni copper. Period 5 Group 7.
A period 4 element is one of the chemical elements in the fourth row or period of the periodic table of the elements. The group is also called the titanium group or titanium family after its lightest member. We have to find the configuration of outer electrons in manganese.
It contains the elements titanium Ti zirconium Zr hafnium Hf and rutherfordium Rf. What would you expect for the configuration of outer electrons of iron. Papers and texts published today have gone over to the newer nomenclature but sometimes we are using older literature.
Period 5 group 2. Periods 4 elements contain potassium calcium scandium titanium vanadium chromium manganese iron cobalt nickel copper zinc and end at krypton. Step 1 of 3.
Period 4 period 4 group 9. They are all transition metals. We are given that manganese belongs to Group VIIB in Period 4.
They are found in trace amounts in the atmosphere in fact 1 of the atmosphere is argon. TYPES OF REACTIONS PROJECT. Arranged this way elements in the same group column have similar chemical and physical properties reflecting the periodic lawFor example the halogens lie in.
Zirconium the second element in Group 4 is in period 5 not period 2. The periodic table is laid out in rows to illustrate recurring trends in the chemical behaviour of the elements as their atomic number increases. Helium He neon Ne argon Ar krypton Kr xenon Xe and radon RnThe name comes from the fact that these elements are virtually unreactive towards other elements or compounds.
Periodic Table of the Elements Period Group 1 IA 1A 18 V IIIA 8A 1 1 H 1008 2 IIA 2A 13 IIIA 3A 14 IVA 4A 15 VA 5A 16 VIA VIIA 6A 17 7A 2 He 4003 2 3 Li 6941 4 Be 9012 5 B 1081 6 C 1201 7 N 1401 8 O 1600 9 F 1900 10 Ne 2018 3 11 Na 2299 12 Mg 2431 3 IIIB 3B 4 IVB 4B 5 VB 5B 6 VIB 6B 7 VIIB 7B. Like other groups the members of this family show patterns in electron configuration especially in the outermost shells resulting in trends in chemical behavior. Group 4 is the second group of transition metals in the periodic table.
Period 5 group 6. A period in the periodic table is a row of chemical elementsAll elements in a row have the same number of electron shellsEach next element in a period has one more proton and is less metallic than its predecessor. Period 5 Group V.
The name comes from the fact that these elements are virtually unreactive towards other elements or compounds. Group 8 is a group column of chemical elements in the periodic table. Potassium left K right Calcium left Ca right Scandium left Sc right.
The group itself has not acquired a trivial name. It belongs to the broader grouping of the transition metals. So technically no element is in Group 4 period 2.

How To Read The Periodic Table Groups Periods Chemtalk

Potassium Is In Group 1 Period 4 Periodic Table Homeschool Science Science Classroom
No comments for "The Element in Period 4 and Group 8"
Post a Comment